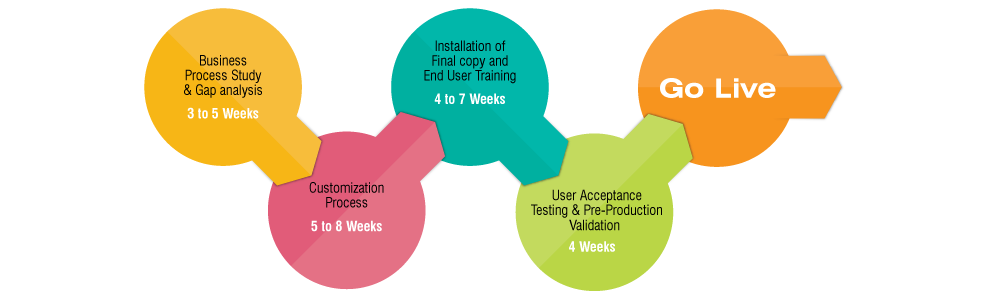
Implementation process starts with study on business process study at STEP. Under this session, MOTTOSYS domain expert will attend for understanding on various procedures (SOPs) and will evaluate all the requirements according to regulatory guidelines. It will help to find out procedural gaps if any, and suggest management for necessary corrections.
Based on process study, a gap analysis session will carryout to find differences between GMPPro processes vs. actual company processes. It will help to access the customization requirements within the scope of GMPPro. A detailed report will be submitted to STEP with all necessary customizations and plan of action on STEP requirements.
During this process, the experts’ will interacts with all the power users from every department and collects all necessary information for possible customizations within the scope of GMPPro. Any requirement other than the scope of GMPPro will be intimated to the management for further activity. Motto Systems will document every process study and will give a detailed report to STEP on every process. Upon review and confirmation from concerned power users, the same will be finalized and taken for customization.
Any change in the process after customization is treated as out of scope and additional work for Motto Systems.
Based on the procedural changes collected during the business process study & GAP Analysis, Motto Systems’ development team start working on various customization requirements to match the process with client SOPs. Under the domain expert’s supervision, an experienced software development team works on all the requirements for desired results. Software QA team performs various test cycles on entire development for error free deployments.
Once all procedures are customized according to the client requirements, an updated copy of application has to be hosted into client server as an executable copy. Along with customization, preliminary training will be provided to all client identified GMPPro users.
Once the copy is ready to the customized needs of STEP, application will be hosted at STEP’s server. A detailed training schedule will be given to all targeted users for this course of implementation. GMPPro training team will give complete knowledge sharing through manual and practical methods.