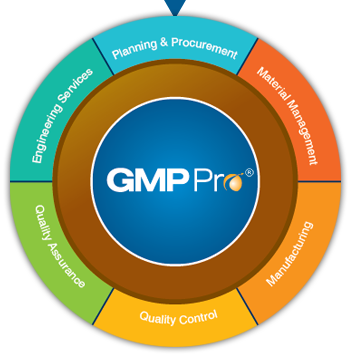
GMPPro is a software product specifically designed and developed for the life science and pharmaceutical industries to manage day to day compliance requirements in Procurement / Stores / Production / Quality Control / Quality Assurance / Manufacturing and Engineering departments.

GMPPro is a highly capable software product that manages pharmaceutical processes with inbuilt cGMPs.
The process requirements from the existing Standard Operating Procedures (SOP) frameworks are incorporated into the GMPPro database. This makes GMP compliance fully automated and seamless.
Additionally, it has a user friendly interface with intuitive controls making it easy to learn and use. It encompasses all production processes starting from raw material procurement to finished product dispatch.
GMPPro has been developed by experts in this field, utilizing the latest technology platforms.
GMPPro inspires enhanced Quality practices from the ground up. Designed with a deeper understanding of processes, GMPPro greatly aids the users in adhering to the standards while the uniquely configured built-in CGMP functions facilitate effective control over operational limitations, deviations and non-conformities.





With electronic data records that streamline all processes of pharmaceutical manufacturing, GMPPro eliminates the drawbacks of manual documentation and re-work, which in turn leads to efficient outcomes for the company.
GMPPro enables the integration of the current active SOP frameworks and quality checks , which, in turn, prevents errors, maximizes quality and assures cGMP compliance. It facilitates easy retrieval of vital data for audit and review purposes.
GMPPro can also generate instant reports enabling the review of the efficacy of procedures in the company at any given point.