

MottoSystems’ prides on a perceptive approach to its work that realizes error free, stringent and easy to implement Quality Management Systems every time. The team of qualified subject matter experts at MottoSystems’ interacts with personnel of relevant departments before setting out to design systems that augment best practices at all levels.

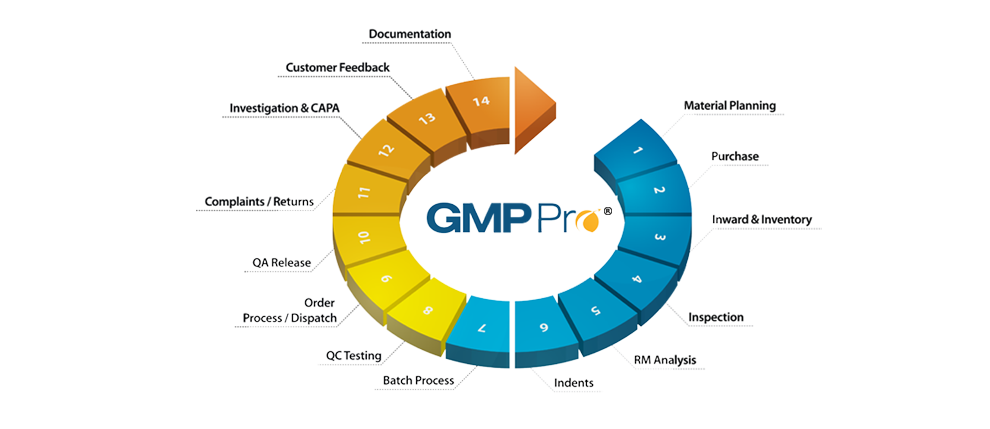
GMPPro ushers stringent compliance regimes where deviations from Standard Operating Procedures become almost extinct. It lends control over document distribution and access, enables supplier control through effective evaluation and effectively reigns in rejections and non-conformities to catalyze overall profitability of the organization.

MottoSystems’ appreciates the need for effective coordination and communication at inter and intra department levels for realizing overall efficiency, and GMPPro achieves this by bringing various functions on to one integrated platform while eliminating silos between departments and the ensuing data duplication for improved data integrity
GMPPro works intelligently for data delegations and validations. By reducing manual dependencies it improves ease of data administration during investigations, review, analysis & trending of data.

GMPPro saves great in terms of both money and time. Not only it disposes off with the onerous manual entries but also acts as your ready reckoner during data vertification & reporting in addition to facilitating timely delivery to customers. Not to mention the cost of production reruns in case of non-compliance to procedures.